本文主要是介绍文献速递:PET-影像组学专题—18F-FDG PETCT影像学的影像组学模型用于预测早期宫颈鳞状细胞癌无病生存率,希望对大家解决编程问题提供一定的参考价值,需要的开发者们随着小编来一起学习吧!
文献速递:PET-影像组学专题—18F-FDG PET/CT影像学的影像组学模型用于预测早期宫颈鳞状细胞癌无病生存率
01
文献速递介绍
宫颈癌是女性死亡的主要原因之一。在早期宫颈癌中,根治性手术加/不加个体化辅助化疗放疗是指南推荐的治疗选择,然而约25%的病例仍会出现复发。一些术后病理风险因素,如肿瘤直径大、深部基质侵犯、淋巴血管空间侵犯(LVSI)、盆腔内侵犯、手术切缘阳性和淋巴结转移,可能会增加复发的发生率。然而,在临床上,即使是没有风险因素的患者或由于病理风险而接受辅助化疗放疗的患者,也可能会有不良的生存情况。
因此,这些因素并不总是能预测患者的生存。因此,有必要开发一个准确和全面的预后预测工具,以补充现有的指南。F-2-氟-2-脱氧-D-葡萄糖正电子发射断层扫描/计算机断层扫描(18F-FDG PET/CT)是一种分子和功能成像方式,广泛用于宫颈癌的诊断、初步分期、反应评估、复发检测和生存分析。传统的PET/CT参数,如最大标准化摄取值(SUVmax)、平均标准化摄取值(SUVmean)、代谢性肿瘤体积(MTV)和总病变糖代谢(TLG),用于反映与临床结果的关系,但未能得出一致的结论。影像组学是一个新兴领域,通过各种技术从定量医学影像中提取特征。影像组学特征可以量化肿瘤的强度、形状和异质性,并已应用于肿瘤检测、诊断、治疗反应和预后。
Lucia等人成功地报告了基于PET/CT和MR的影像组学模型,用于预测晚期宫颈癌的复发,并进行了进一步的外部验证。这个影像组学模型的预测准确性高达90%,而使用标准临床变量的预测准确性为56–60%。可能由于随访时间较短,目前在早期宫颈癌中使用PET/CT影像组学特征进行的预后研究很少。
此外,由于宫颈癌最常见的病理类型是鳞状细胞癌,我们的研究是在具有相同组织学类型的同质患者群体中进行的,这增加了我们发现的普遍性。因此,我们打算开发一个PET/CT影像组学模型,以准确预测早期宫颈鳞状细胞癌患者的预后。
Title
题目
Radiomics model of 18F-FDG PET/CTimaging for predicting disease-free survival of early-stage uterine cervical squamous cancer 18F-FDG PET/CT
18F-FDG PET/CT影像学的影像组学模型用于预测早期宫颈鳞状细胞癌无病生存率
Abstract-Background
摘要-背景
To explore an effective predictive model based on PET/CT radiomics for the prognosis of early-stage uterinecervical squamous cancer.
探讨基于PET/CT影像组学的有效预测模型,用于早期宫颈鳞状细胞癌的预后。
Methods
方法
Preoperative PET/CT data were collected from 201 uterine cervical squamous cancer patients with stage IB-IIAdisease (FIGO 2009) who underwent radical surgery between 2010 and 2015. The tumor regions were manually segmented,and 1318 radiomic features were extracted. First, model-based univariate analysis was performed to exclude features with smallcorrelations. Then, the redundant features were further removed by feature collinearity. Finally, the random survival forest (RSF)was used to assess feature importance for multivariate analysis. The prognostic models were established based on RSF, and theirpredictive performances were measured by the C-index and the time-dependent cumulative/dynamics AUC (C/D AUC).
从201名处于IB-IIA期(FIGO 2009)的宫颈鳞状细胞癌患者中收集了术前PET/CT数据,这些患者在2010年至2015年间接受了根治性手术。肿瘤区域手动分割,提取了1318个影像组学特征。首先,进行基于模型的单变量分析,以排除相关性小的特征。然后,通过特征共线性进一步移除冗余特征。最后,使用随机生存森林(RSF)评估多变量分析中的特征重要性。基于RSF建立了预后模型,其预测性能通过C指数和时间依赖的累积/动态AUC(C/D AUC)进行衡量。
Results
结果
In total, 6 radiomic features (5 for CT and 1 for PET) and 6 clinicopathologic features were selected. The radiomic,clinicopathologic and combination prognostic models yielded C-indexes of 0.9338, 0.9019 and 0.9527, and the mean values of theC/D AUC (mC/D AUC) were 0.9146, 0.8645 and 0.9199, respectively.
总共选取了6个影像组学特征(CT 5个,PET 1个)和6个临床病理特征。影像组学、临床病理和组合预后模型的C指数分别为0.9338、0.9019和0.9527,其平均时间依赖的累积/动态AUC(mC/D AUC)分别为0.9146、0.8645和0.9199。
Conclusions
结论
PET/CT radiomics could achieve approval power in predicting DFS in early-stage uterine cervical squamouscancer.
Keywords: 18F-FDG PET/CT, radiomics, DFS, prediction, early-stage uterine cervical squamous cancer
PET/CT影像组学在预测早期宫颈鳞状细胞癌的无病生存率(DFS)方面能够取得认可的效力。
Figure
图
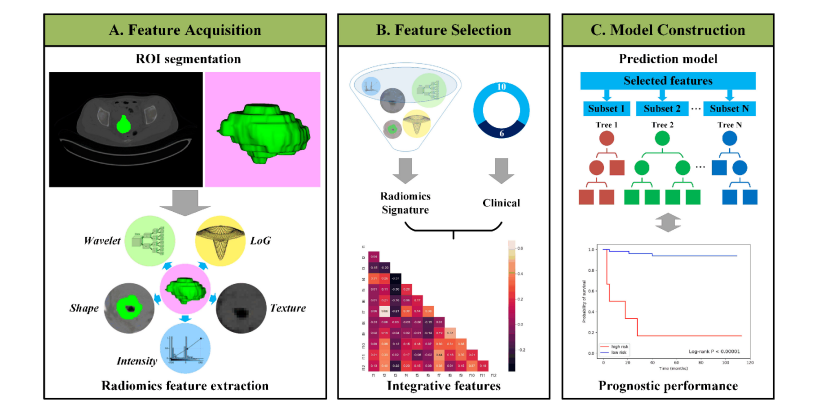
Fig. 1. Outline of the workflow from data input, feature extraction, selection, and model construction.
图1. 数据输入、特征提取、选择和模型构建的工作流程概述
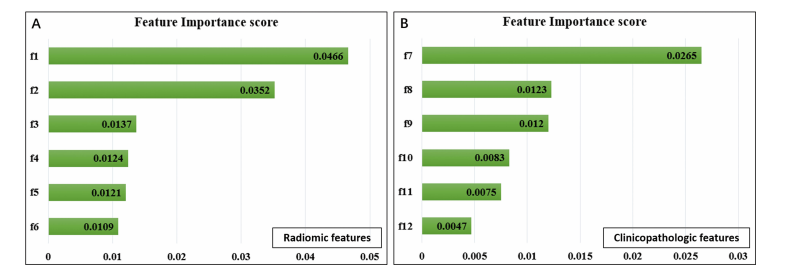
Fig. 2. Selected radiomic features in predicting DFS. Feature importance of radiomic (A) and clinicopathologic features (B). f1, CT_wavelet *HLH_gldm_SmallDependenceHighGrayLevelEmphasis; f2, CT_log-sigma-4-0-mm-3D_glrlm_RunVariance; f3, CT_log-sigma-4-0-mm-3D_**gldm_LowGrayLevelEmphasis;f4,CT_log-sigma-5-0-mm-3D_glrlm_LongRunHighGrayLevelEmphasis; f5, CT_log-sigma-5-0-mm-3D_gldm_*DependenceNonUniformity;f6,PET_original_glcm_SumSquares; f7, TLG; f8, LVSI; f9, lymph node metastasis; f10, deep stromal invasion; f11, preoperative SCCA level; f12, FIGO stage.
图2. 预测无病生存率时选定的影像组学特征。影像组学(A)和临床病理特征(B)的重要性。f1, CT小波HLH_gldm小依赖高灰度强调;f2, CT对数-西格玛-4-0毫米-3D_glrlm运行方差;f3, CT对数-西格玛-4-0毫米-3D_gldm低灰度级强调;f4, CT对数-西格玛-5-0毫米-3D_glrlm长运行高灰度级强调;f5, CT对数-西格玛-5-0毫米-3D_gldm依赖非均匀性;f6, PET原始glcm_平方和;f7, TLG;f8, LVSI;f9, 淋巴结转移;f10, 深部基质侵犯;f11, 术前SCCCA水平;f12, FIGO分期。
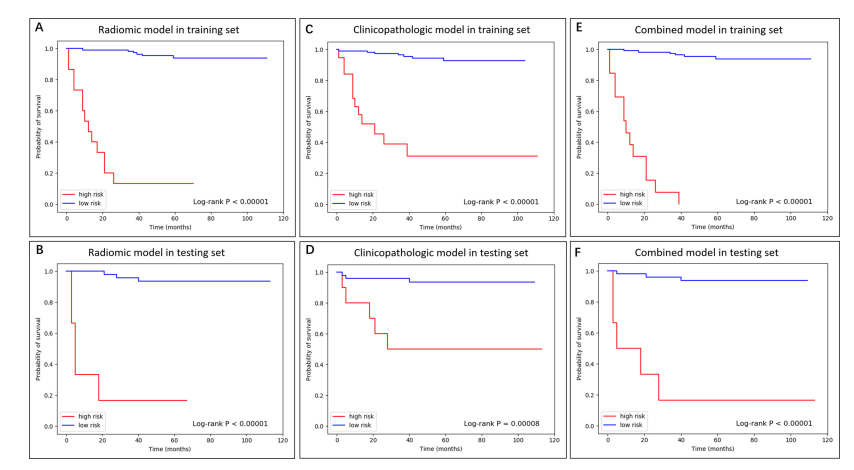
Fig. 3. The K-M curve of radiomic, clinicopathologic, and combined model in training and testing dataset.
图3. 训练和测试数据集中影像组学、临床病理和组合模型的K-M曲线。
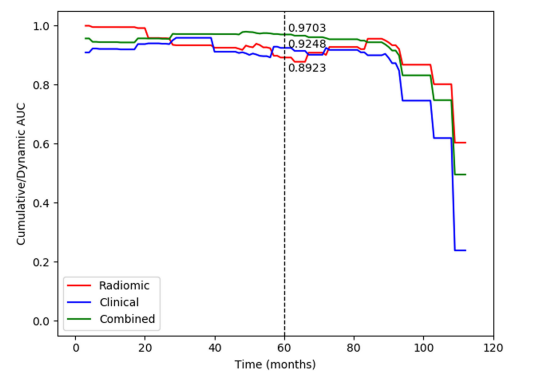
Fig. 4. The C/D AUC curve of the radiomic, clinicopathologic, and combined models in the testing dataset. As an indicator to measure thepreoperative predictive efficiency of the model in addition to the C-index, the mC/D AUC was further calculated over all time ranges. The curveshowed that the combined model achieved the best performance during the research period, and the mC/D AUCs of the radiomic, clinicopathologicand combined models were 0.9146, 0.8645, and 0.9199, respectively. At 60 months after surgery, the preoperative combined model showed ahigher predictive value with a C/D AUC60 of 0.9703 compared to 0.9248 in the clinicopathologic model and 0.8923 in the radiomic model (tagline shown). However, the predictive ability was obviously decreased after 100 monthsThe predictive ability was obviously decreased after**100 months.
图4. 测试数据集中影像组学、临床病理和组合模型的C/D AUC曲线。作为除C指数外衡量模型术前预测效能的指标,对所有时间范围计算了mC/D AUC。曲线显示,在研究期间,组合模型表现最佳,影像组学、临床病理和组合模型的mC/D AUC分别为0.9146、0.8645和0.9199。手术后60个月时,术前组合模型显示出更高的预测价值,其C/D AUC60为0.9703,相比之下临床病理模型为0.9248,影像组学模型为0.8923(如标线所示)。然而,预测能力在100个月后明显下降。
Table
表
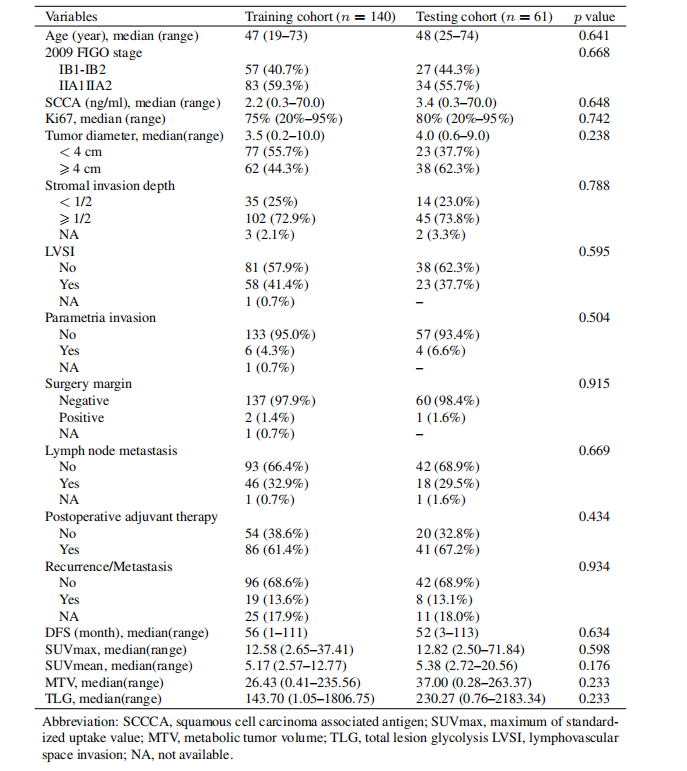
Table 1 Clinicopathologic characteristics
表1临床病理特征
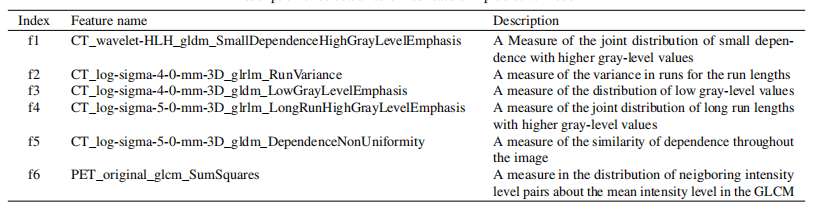
Table 2 Description of selected radiomics feature in predictive model
表2 预测模型中选定影像组学特征的描述
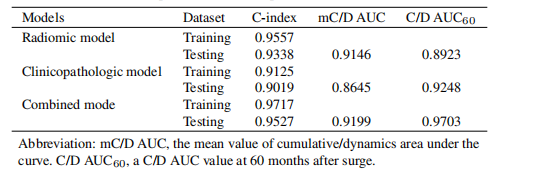
Table 3The performance of the predictive model
表3预测模型的性能
这篇关于文献速递:PET-影像组学专题—18F-FDG PETCT影像学的影像组学模型用于预测早期宫颈鳞状细胞癌无病生存率的文章就介绍到这儿,希望我们推荐的文章对编程师们有所帮助!







